Un próximo ensayo clínico en Massachusetts General Hospital y otros dos hospitales de Massachusetts evaluará la eficacia del medicamento favipiravir en el tratamiento de pacientes con el COVID-19. Cops said they are trying to find out for how long Mishra had been making fake drugs.

Favipiravir 200mg Tablet Glenmark Pharmaceuticals 5 10 Tabs Rs 1030 Strip Id 22401340930
Like the experimental antiviral drugs T-1105 and T-1106 it is a pyrazinecarboxamide derivative.

Favipiravir covid fda. Meanwhile the analytical report of samples of Hydroxychloroquine Sulphate tablets M HCQ 200 manufactured by Max Relief. As per reports the drugs consignment packed in 170 boxes was seized from a chemist shop located at Kanika Chhak in the millennium city. The reported CLF for favipiravir 1600 mg dosed once daily is 298 Lhr 030 and the CLF values for favipiravir 600 mg dosed twice daily on days 1-2 and once daily on days 3-7 were 672 Lhr 168 on Day 1 and 289 Lhr 091 on Day 7.
The case has sparked concern among activists on spurious drugs that have reached patients. A special Drugs Control squad on Friday claimed to have seized nearly 17000 counterfeit anti-viral drug Favipiravir prescribed for Covid treatment following a raid in Cuttack. Boris Juelg MD PhD investigador principal del estudio en Mass General explica cómo se desarrolló la droga y lo que se sabe actualmente sobre su potencial para el tratamiento.
Glenmark Pharmaceuticals on Tuesday announced its interim data of 503. Favipiravir has been widely used in treatment of COVID. On May 10 2021 the FDA amended the EUA to.
It has already proved its safety profile as it has received FDA. By Rajendra Diwe. From medical staff to common people recently published in the Brain Behavior and Immunity motivated us to pen down a concise yet informative viewpoint entitled COVID-2019-suicides.
There is a test that will check for seasonal flu type A and B viruses and SARS-CoV-2 the virus that causes COVID-19. 600 mg twice daily. An inspiring editorial by Montemurro 2020 entitled The emotional impact of COVID-19.
The accused have been identified as Sandeep Mishra. 1600 mg twice daily. New Delhi May 25 IANS.
FabiFlu otherwise known as Favipiravir in India was found to be safe and effective in Covid-19 patients reveals a new study. The drug has been widely used for Covid-19 treatment in the country. The Food and Drugs Administration FDA seized Favimax 400 and 200 Favipiravir tablets and hydroxychloroquine tablets worth Rs 15 crore.
Diagnostic testing can help determine if you are sick with flu COVID-19 or another respiratory infection. Is there a test that can detect both flu and COVID-19. The city police have arrested a man for allegedly manufacturing COVID-19 drug Favipiravir at a private lab in Meerut district of Uttar Pradesh.
From early 2020 through 2021 several hundred drug companies biotechnology firms university research groups and health organizations were developing therapeutic candidates for COVID-19 disease in various stages of. Favipiravir sold under the brand name Avigan among others is an antiviral medication used to treat influenza in Japan. The six samples seized by Maharashtra FDA in the raids on three premises in Mumbai were found spurious by public analysts after lab analysis.
India on early Wednesday received a consignment of 15 ventilators and 12000 tablets of Favipiravir from Iceland as the country combat the second wave of Covid-19 pandemic. FDA had raided three wholesale. Besides 17755 oxygen concentrators 16301 oxygen cylinders 19 oxygen generation plants 13449 ventilators or BiPAP have also been received from different countries and.
The recommended oral dosing regimen for favipiravir is as follows. COVID-19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus disease 2019 COVID-19. 2481026 is the fearsome and huge number of COVID-19 cases with.
In the last 29 days India has received 12 lakh Favipiravir tablets and 69 lakh Remdesivir vials from various countries as part of international cooperation of Covid-19 relief medical supplies. According to a source from FDA As per a confidential report the two brands Favimax 400 Favipiravir Tablets 400 Favimax 200 Favipiravir Tablets 200 are being sold on-line to the customers across the country. Among these medicines Favipiravir is considered a broad-spectrum antiviral with the spectrum of activity noted against a wide range of RNA viruses a good oral antiviral drug with 97 bioavailability.
A global psychological pandemic. 24 x 7 Technical Support and Call Center Access are available from Micromedex Support. It is also being studied to treat a number of other viral infections including SARS-CoV-2.
Key content Favipiravir found missing in Favimax tablets for treating COVID-19 By ruchika Published On 2021-06-17T1100240530 Updated On 2021-06-17T1716030530 It was informed that Favipiravir a key content was not found in the seized Favimax tablets Agrawal said adding the allegedly fake tablets were supplied by a firm. 13033544100 press option 2. A Randomized Controlled Study on the Safety and Efficacy of Maraviroc andor Favipiravir vs Currently Used Therapy in Severe COVID-19 AdultsCOMVIVIR Trial.
The Government analyst declares samples of brand of Hcq 200 manufactured by non-existing Max Relief Healthcare as Spurious Maharashtra FDA seized a stock of Rs 154 crore of spurious Favimax 400 and 200 mg from the stock points. The FDA granted Emergency Use Authorization EUA for BNT162b2 on December 11 2020 for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 16 years of age and older. It is being developed and manufactured by Toyama Chemical a subsidiary of Fujifilm.
National News Bureau Of Thailand

Thai Made Favipiravir In Human Trials Ahead Of Production

Large North American Favipiravir Pivotal Phase 3 Clinical Trial Expands To Mexico And Brazil

Fujifilm Boosts Production Capacity For Potential Covid 19 Drug Avigan
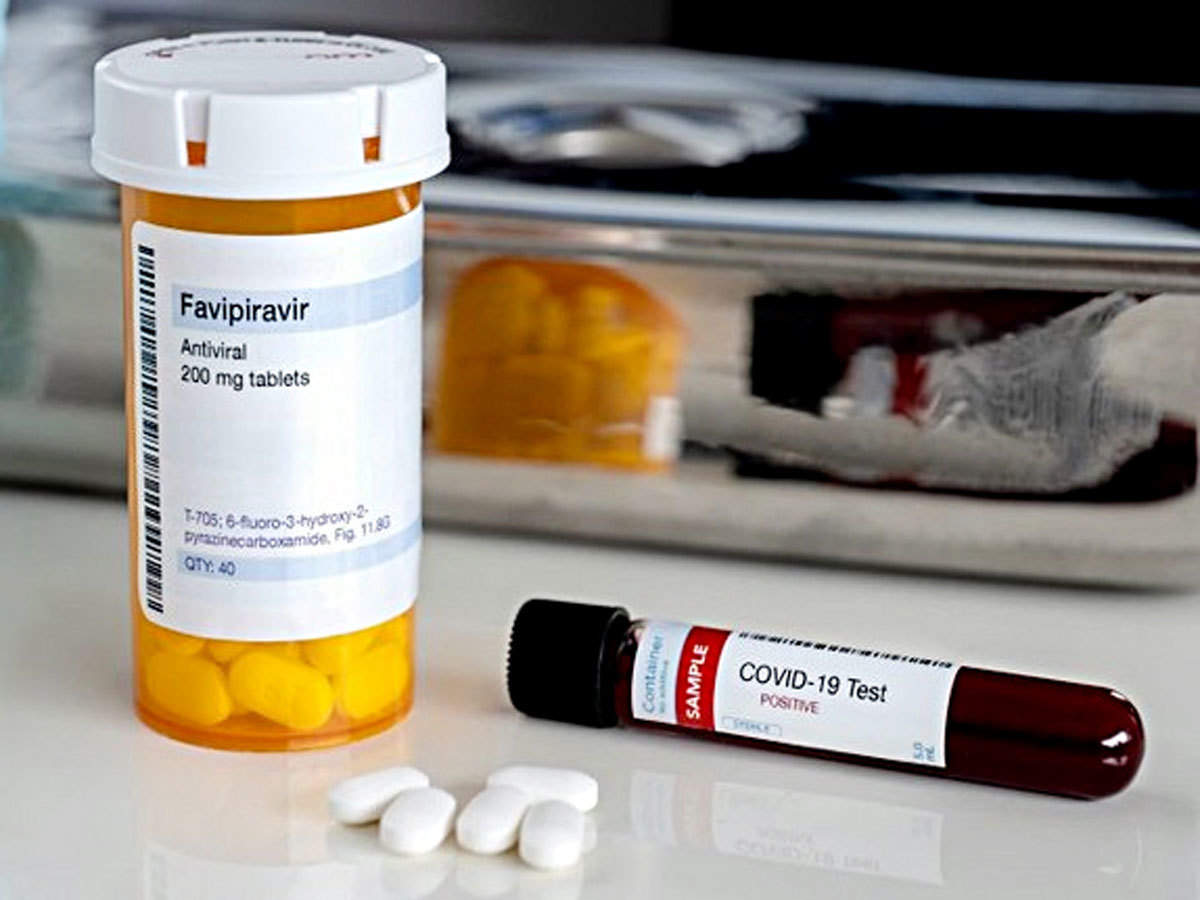
Covid 19 Flu Drug Favipiravir Shows Promise In Reining In Covid 19 Health News Et Healthworld
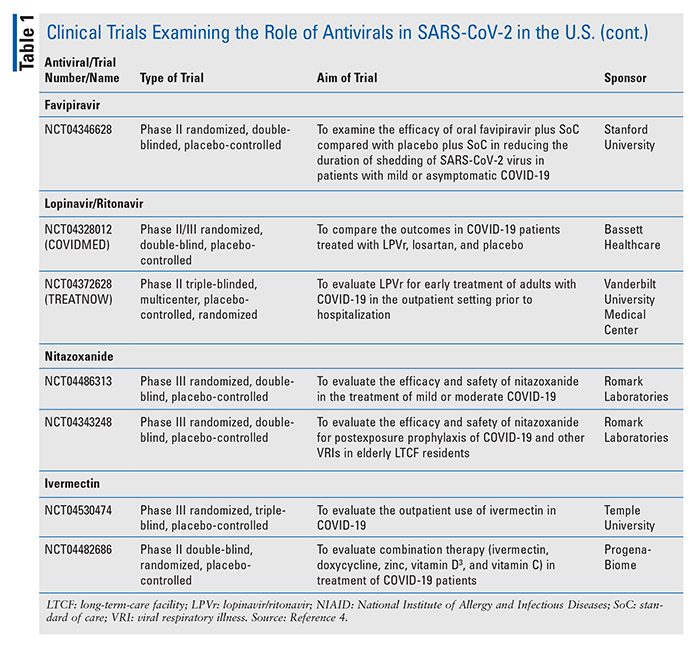
Investigational Antivirals For Managing Covid 19

A New Generic Favipiravir Version In India A Covid 19 Early Stage Treatment For The Lower

China And South Korea Split Over Japanese Anti Flu Drug Avigan In Fight Against Coronavirus South China Morning Post

Pr Thai Government On Twitter Favipiravir Has Been Used For Some Time To Treat Covid19 Patients In Thailand As A Result The Government Pharmaceutical Organization Has Now Produced The Medicine Locally And

Glenmark Starts Phase Iii Favipiravir Combination Trial For Covid 19

Thai Made Favipiravir Tablets For Covid Treatment Get Fda Approval The Star

Favipiravir At High Doses Works To Curb Covid 19 In Hamsters Hcq Ineffective Study Technology News Firstpost
Overview Of Potential Treatments For Covid 19 Aptitude Health

Appili Expands Favipiravir Trial To Long Term Care Facilities In Us

Favipiravir By Fujifilm May Treat Covid 19 Say Chinese Officials

Us Beginning Trials Of Japanese Anti Viral Drug As Potential Covid 19 Treatment

Stanford Researchers To Assess Favipiravir In Covid 19 Outpatients

Fda Clears Favipiravir For Covid 19 Facility Outbreak Prevention Study

The Dhaka Trial Clear Cut Evidence Favipiravir Effective Against Covid 19 With Compelling Results

